Recently, a research team led by Professor Yonghong Deng (Materials Science and Engineering, SUSTech) propose a viable pathway of realizing high-energy-density and high-stability lithium metal batteries by adopting self-organized core-shell composite anodes. The cutting-edge findings were published in Advance Materials (IF = 27.3), entitled “500 Wh/Kg Class Li Metal Battery Enabled by Self-Organized Core-Shell Composite Anode”.
In previous reports, most of reported approaches often seek to suppress dendrites formation at the expense of energy density of lithium metal batteries. In comparison, this work provides a novel scheme that simultaneously stabilizes Li metal deposition from the dendrite and dead lithium formation while reduces the overall inert weight and volume at the cell level, which leads to the successful fabrication of lithium metal full batterie with unprecedented energy density of over 500 Wh/Kg.
In specific, a self-organized core-shell composite anode containing an outer sheath of lithiated liquid metal (LixLMy) and an inner core of Li metal is reported. Benefiting from the composite anode that prevents the dendrite formation and eliminates the use of copper as substrates, the assembled full batteries made of such lithium composite anode with commercially available high-voltage cathode could deliver ultrahigh energy density of exceeding 500 Wh Kg–1 based on the total mass of all the cell components including electrodes, current collectors, separator, electrolyte, and encapsulation materials.
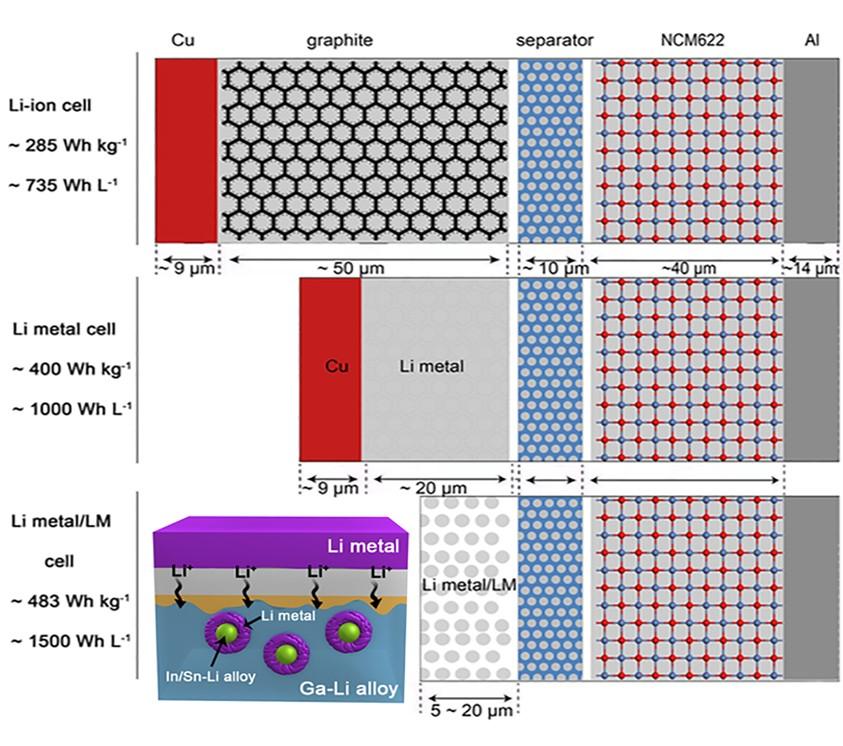
Lithium (Li) metal offers the highest projected energy density as a battery anode; however, its extremely high reactivity induces dendrite growth and dead Li formation during repeated charge/discharge processes, resulting in poor reversibility and catastrophic failure. Approaches reported to date often seek to suppress dendrites formation at the expense of energy density.
Deng’s team reported a strategy that resolves the above conflict and achieves a dendrite-free and long-term reversible Li metal anode. A self-organized core-shell composite anode, comprising an outer sheath of lithiated liquid metal (LixLMy) and an inner layer of Li metal, is developed, which processes high electrical and ionic conductivity and physically separates Li from the electrolyte. The introduction of LixLMy not only prevents the dendrite formation but also eliminates the use of copper as the inert substrates. Full cells made of such composite anodes and commercially available LiNi0.6Co0.2Mn0.2O2 (NCM622) cathodes deliver ultrahigh energy density of 1500 Wh L–1 and 483 Wh kg–1 based on the volumes and masses of every cell component. The high capacity can be maintained for more than 500 cycles, with a fading rate of less than 0.05% per cycle. Paring with LiNi0.8Co0.1Mn0.1O2 (NCM811) further raises the energy density to 1732 Wh L–1 and 514 Wh kg–1.
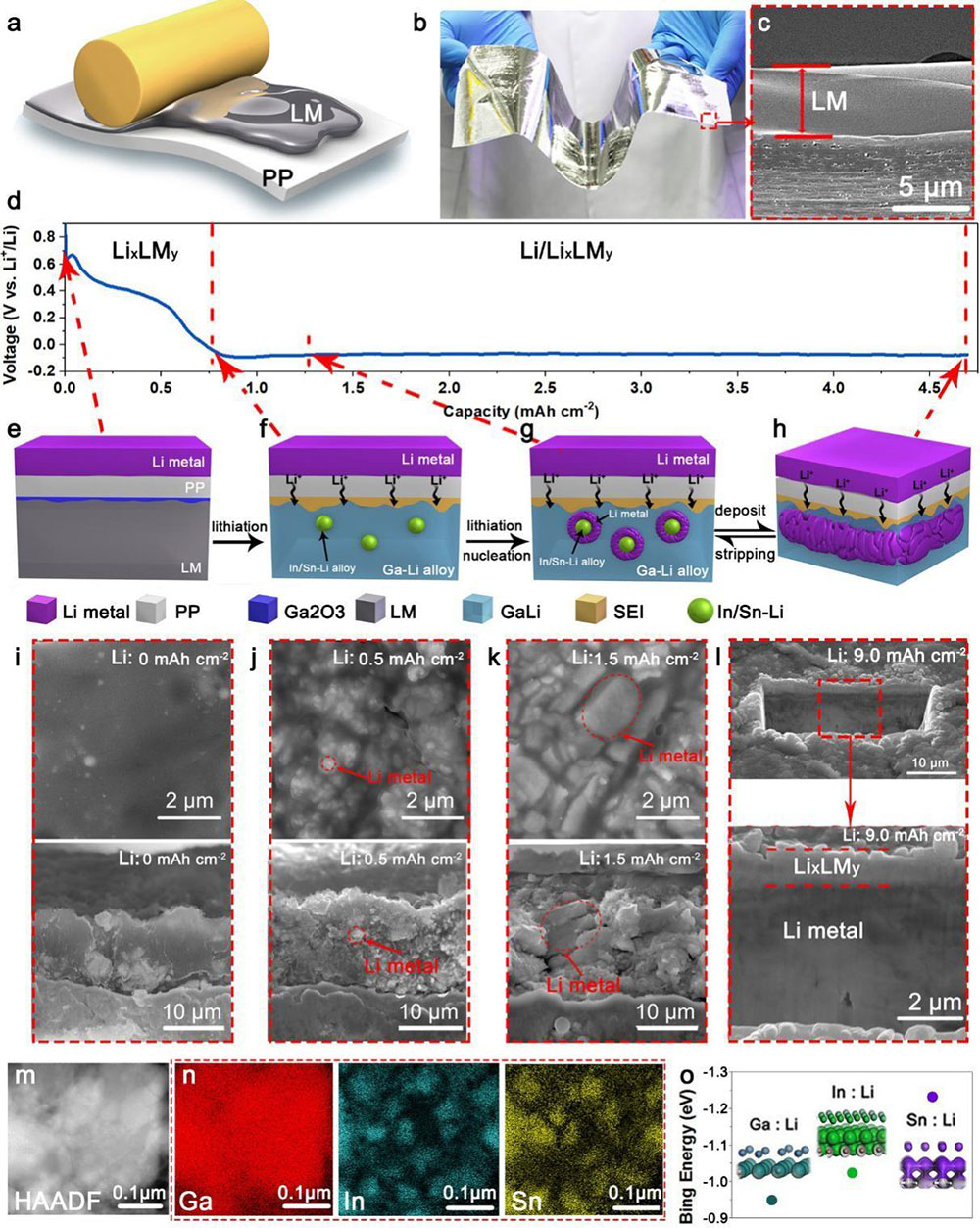
Figure 1. Formation of the composite electrode (Li/LixLMy).
The self-organized composite electrode, Li/LixLMy hereafter, is formed through a one-step lithiation of LM. In brief, a 5 mm thick LM (GaInSn) was blade-coated onto a commercially available polypropylene (P.P.) separator under ambient conditions (Figure 1a). Thanks to LM’s excellent fluidity, the coating was compact and uniform on P.P. (Figure 1b and 1c), and the film can be easily prepared on a large scale (Movie S1, Supporting Information). Assembling a cell consisting of LM/PP and Li foil followed by discharging leads to the spontaneous formation of Li/LixLMy (Figure 1d). A thin layer of Ga2O3 was found on the interface between P.P. and LM due to the rapid oxidation of Ga in LM (Figure 1e). The alloying of LM with Li results in LixLMy at the early stage of the lithiation process (Figure 1f and 1i), evidenced by the drop of discharge voltage from 1.0 V to 0.01 V (Figure. 1d and Figure S1, Supporting Information). The alloy becomes saturated when Li/LM ratio reached ~2.5 (Figure S1, Supporting Information).
Interestingly, spontaneous phase separation occurred in the LixLMy. High-angle annular dark-field (HAADF) images and the energy-dispersive X-ray spectroscopy (E.D.S.) mapping of scanning transmission electron microscopy (STEM) revealed uniform distribution of Ga, Sn, and In before lithiation (Figure S2, Supporting Information), but after lithiation, isolated Sn/In-rich islands embedded in the Ga-rich matrix were found (Figure 1m and 1n), which can be attributed to the higher binding energy of Li to Sn/In than to Ga (Figure 1o). Consequently, Li preferentially binds to Sn/In to form SnInLi clusters, which phase-separate from the GaLi matrix (Figure 1f and 1n)—further fusing Li into LM until saturation resulted in the spontaneous nucleation of Li on the SnInLi clusters (Figure 1g and 1j). With the increasing amount of deposited Li, the nano-size nuclei of Li0 grew to micro-size particles (Figure 1k) and gradually merged into a continuous Li0 thin film (Figure 1h and 1l). Note that the GaLi sheath fully encased all the Li0.
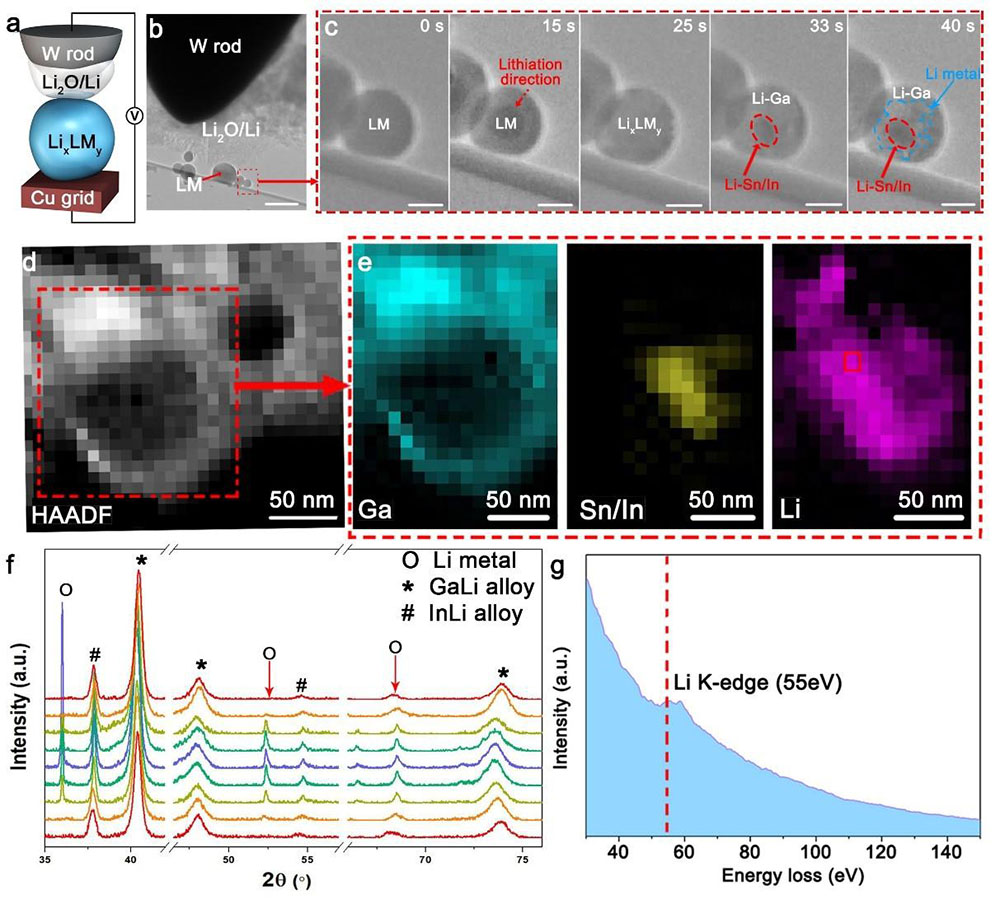
Figure 2. Operando T.E.M. and X-ray study of the self-organization process of forming Li/LixLMy.
Operando T.E.M. was performed to observe the formation of Li/LixLMy directly. The custom-made S.T.M. holder consists of a tungsten needle electrode coated with Li and droplets of LM on a copper grid with carbon support (Figure 2a and 2b). As common practice adopted by the in-situ T.E.M. technique, the native Li2O on the Li surface serves as a solid electrolyte. The needle was brought into contact with the LM to lithiate the latter. The outer area of the LM became brighter after 15 s lithiation, and the entire LM particle was fully saturated with Li to form LixLMy at 25 s (Figure 2c and Movie S2, Supporting Information). After that, Li0 started to nucleate (33 s) and grow (40 s) in the matrix of GaLi, forming Li/LixLMy. HAADF and electron energy loss spectroscopy (EELS) analysis further confirmed the existence of Ga-rich phase in the peripheral regions of Li/LixLMy and the Sn/In-rich phase and Li in the middle of Li/LixLMy (Figure 2d, 2e, and 2g). The dark spots represent Sn and In in the red dotted region of 33 s and 40 s of Figure 2c, and the bright spots around the dark spots should be Li0 in the blue dotted region of 40 s of Figure 2c, which also corresponds to EELS mapping in Figure 2e. The ex-situ and operando studies both confirm the self-organized composite structure.
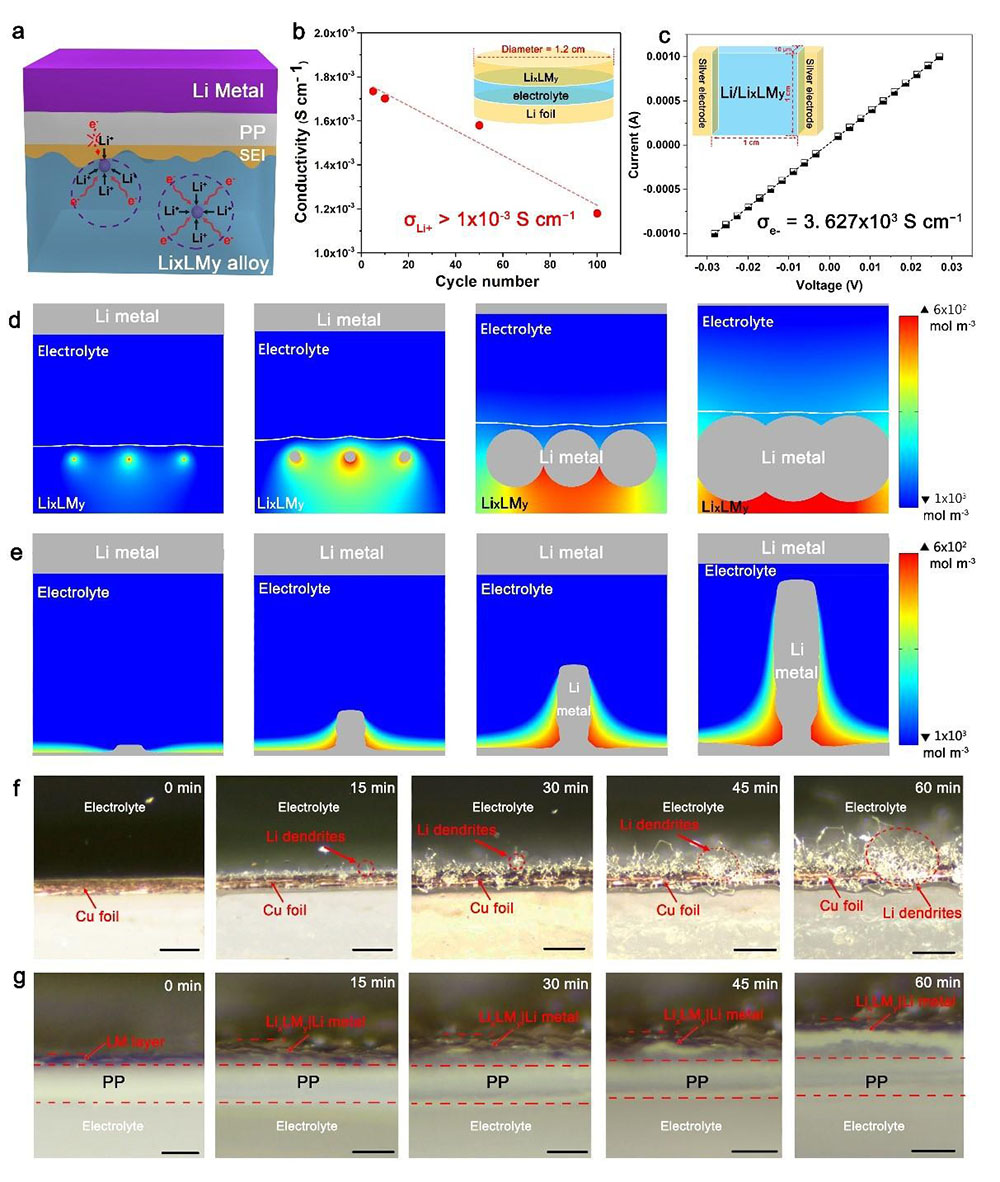
Figure 4. Study of the dendrite-free characteristic of Li/LixLMy.
The absence of dendrite in Li/LixLMy benefitted from combining two factors: a conductive protection layer and an effective physical barrier. First of all, the GaLi matrix provides an environment of high ionic and electrical conductivity, eliminating the fundamental cause for dendrite and dead lithium, i.e., the inhomogeneity in both chemistry and morphology of the S.E.I. on the lithium surface. Such a conductive layer evens out the opportunity for lithium deposition; thus, the isotropic nucleation and homogenous growth of Li occurs (Figure 4a). The ionic conductivity of the GaLi sheath, which was deduced from the impedance analysis, remained at a high value of large than 1.0×10−3 S cm−1 over cycling (Figure 4b and Figure S5, Supporting Information), indicating fast Li+-transportation kinetics in GaLi. The electrical conductivity of GaLi, measured by the standard van der Pauw method, also showed a high value of 3.63×103 S cm−1 (Figure 4c). By taking the conductivity parameters, we also simulated the deposition of Li in LixLMy and on conventional Cu foil using COMSOL. For the former, the small Li nucleus appeared in the matrix of LixLMy and then grew into larger particles before merging into a continuous Li0 thin film (Figure 4d).
Conversely, a small Li protrusion appeared on the Cu foil and immediately grew to a large pillar-like Li whisker (Figure 4e). These simulation results agree well with the operando optical microscopy study (Figure 4f and 4g) of plating Li onto different substrates. Direct plating of Li onto Cu foil for 60 min at a current density of 2.0 mA cm−2 resulted in the continuous formation of a large number of Li dendrites (Figure 4f). In sharp contrast, no dendrite was observed when Li was plated into LM(Figure 4g, Figure S6, and Movie S3, Supporting Information). Instead, the LM expanded and contracted with the repeated lithiation and de-lithiation of LixLMy, demonstrating the high elasticity of the LixLMy sheath.






