Harvesting electricity from renewable energy sources is an ongoing goal for the world working to move away from fossil fuels. The ability to use electrons derived from renewable energy to drive chemical transformations offers opportunities for the sustainable production and regeneration of carbon-based chemicals and fuels.
Research led by scholars at Southern University of Science and Technology (SUSTech) may have provided a new pathway for the production of carbon-based chemical and fuels from renewable energy.
Professor Yongye Liang (Materials Science and Engineering) and Associate Professor Yanggang Wang (Chemistry) brought their research teams together with scholars from the School of Chemical, Biological, and Environmental Engineering at Oregon State University. Together, they have collaborated to significantly improve the electrocatalytic reduction of carbon dioxide. The paper outlining their success was published in the high-impact academic journal, Nature Energy (IF = 46.5), under the title of “Molecular engineering of dispersed nickel phthalocyanines on carbon nanotubes for selective CO2.”
The carbon cycle plays an essential role in the Earth. The increasing use of fossil fuels has seen the concentration of carbon dioxide (CO2) in the atmosphere increase substantially. There has been a similar increase in environmental problems facing the world as a result. The electroreduction of CO2is a possible way to reduce carbon emissions and to produce carbon-based fuels and chemicals. Electrocatalyst performance and system cost are vital for the applications of this technology.
Current technology shows the electroreduction of CO2to carbon monoxide (CO) as a relatively high product selectivity and energy conversion efficiency. The state-of-the-art catalysts are gold (Ag) and silver (Au)-based materials. However, they are costly, which limits their commercial applications. Single-atom catalysts (SACs) are emerging candidates with high activities. Of particular note are nickel (Ni) based SACs, due to their high CO selectivity.
One of the challenges is that the pyrolysis synthesis of SAC, which is the synthesis brought about by the decomposition of precursors at high temperature, results in complicated active reaction sites. That current methodology leads to low product selectivity at the optimal current densities, complicating performance optimization.
Yongye Liang’s research group had previously studied cobalt phthalocyanine (CoPc)/carbon nanotube (CNT) hybrid structures. They had found that the CNT hybrid displayed better catalytic performance than CoPC molecules in CO2reduction electrocatalysis. Their following research applied their methodology to reveal the fundamental activities of metal phthalocyanines, such as iron (Fe), cobalt (Co) and manganese (Mn).
In theNature Energyarticle, the research groups found that molecularly dispersed electrocatalysts (MDEs) can be built by anchoring metal phthalocyanine molecules onto the sidewalls of CNTs. They showed a similar structure to Ni SACs (Fig. 1). Even though it showed high CO product selectivity than the CoPc system and pyrolyzed Ni SAC, their nickel phthalocyanine (NiPc) MDE was unstable under the electroreduction of CO2. That limits its commercial applications.
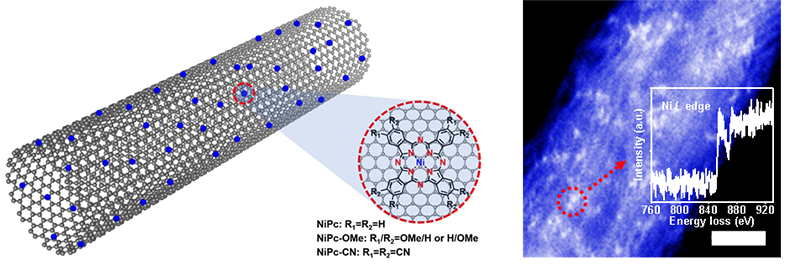
Fig. 1. Schematic presentation of the NiPc-MDEs and Z-contrast HADDF-STEM image of NiPc-OMe-MDE
The research groups used molecular engineering to tune the performance of the MDE catalysts. They found that they could get a more stable and highly selective catalyst by introducing functional groups in the right place. It also provides better electrical performance across multiple measures to afford high reduction current densities of several hundreds of milliampere per square centimeter. It set new records of noble-metal-free and molecular-based CO2RR electrocatalysts (Fig. 2).
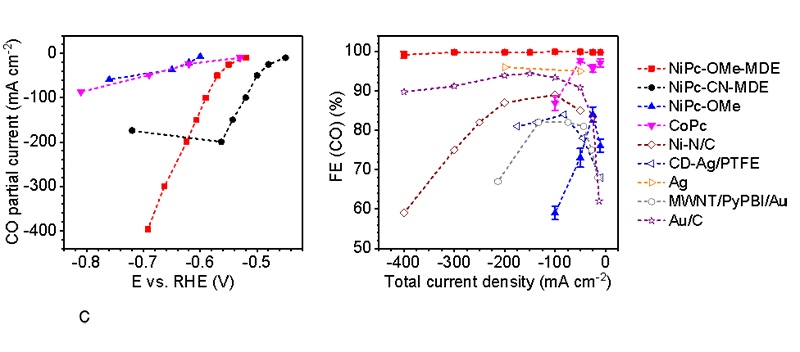
Fig. 2. The electrocatalytic performance of NiPc-OMe MDE in the gas diffusion electrode
MDEs with well-defined active centers also facilitated their understanding of the underlying mechanism on structural factors that affected the electrocatalytic performance. The research groups conductedin-situ/operandoX-ray absorption spectroscopy and theoretical calculation studies.
The initial activity correlated with the partial reduction of Ni centers alongside the reduction of the catalyst molecules. It can be improved by cyano group functionalization. Catalytic stability can be improved by strengthening Ni-N bonds and assisting the CO desorption (Fig. 3).
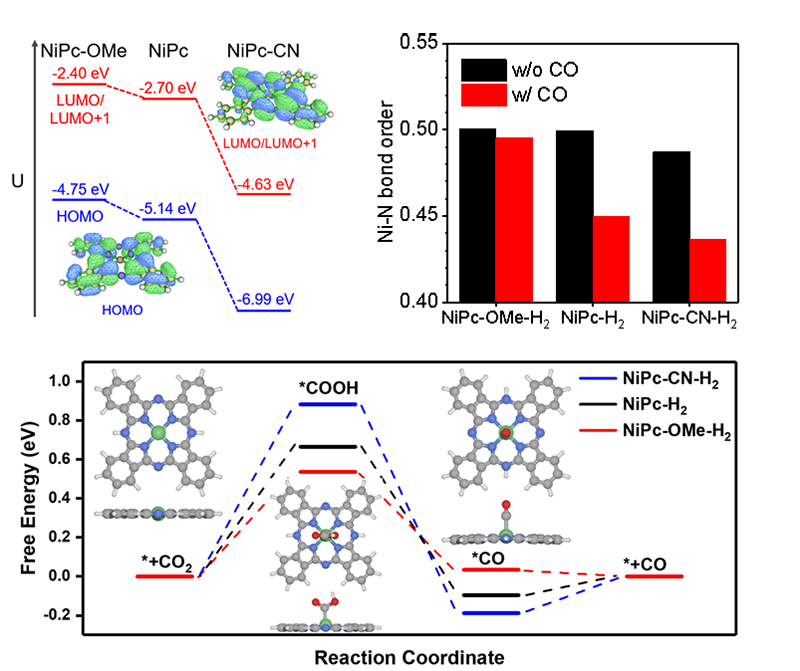
Fig 3. DFT calculations of NiPcs catalyzing CO2RR
These studies pave a new path for the development of high-performance electrocatalysts. They also provide an insight into our understanding of the structure-property relationship of electrocatalysis. Due to their high-performance, NiPc-OMe-MDE could be readily incorporated into practical CO2RR electrolyzers.
Dr. Xiao Zhang, Yang Wang, and Associate Professor Meng Gu (SUSTech) and Maoyu Wang (Oregon State) are the co-first authors of the paper. Co-corresponding authors are Yongye Liang, Yanggang Wang, and Oregon State University Assistant Professor Zhenxing Feng. Additional contributions came from Yale University, Argonne National Laboratory, Northwestern University, Stanford University, and Tsinghua University.
This work was partially supported by Shenzhen Fundamental Research Funding, Guangdong-Hong Kong-Macao Joint Laboratory for Photonic-Thermal-Electrical Energy Materials and Devices, and Guangdong Provincial Key Laboratory of Catalysis.






