Prof. Yongye Liang’s research group has developedhighly selective and active electrocatalysts for carbon dioxide reduction.This work has been recently published in the journal “Nature Communications” entitled “Highly Selective and Active CO2Reduction Electrocatalysts Based on Cobalt Phthalocyanine/Carbon Nanotube Hybrid Structures”.
Global carbon cycle is one of the keys for making the Earth sustainable. However, human activities in the past centuries have significantly increased the amount of CO2in the atmosphere. The electrocatalyticreduction of CO2to useful carbon based products is a promising avenue. It can work under ambient condition in water and be coupled with renewable energy sources like solar energy.Currently, electroreduction of CO2suffers from high overpotential of the operation and low selectivity of the desired product. Cost-effective electrocatalysts with high activity, selectivity and good stability are highly desirable to make the CO2elecroreduction process industrially viable.
Liang and his coworkers reported a cobalt phthalocyanine (CoPc) based high-performance CO2reduction electrocatalyst material developed with a combined nanoscale and molecular approach. On the nanoscale, CoPc molecules are uniformly anchored on carbon nanotube (CNT) walls with strong pi-pi interaction between them to form the CoPc/CNT hybrid, affording substantially increased current density and improved catalytic selectivity and stability compared to CoPc for electroreduction of CO2to CO(an importantindustrial gasthat has many applications in bulk chemicals manufacturing). The CoPc/CNT hybrid catalyst shows a high and stable current density of around 10 mA cm-2with a FE of over 90% for CO2reduction to CO at an overpotential of 0.52 V in 0.1 M KHCO3aqueous solution for over 10 hours. On the molecular level, the catalytic performance is further upgraded by introducing cyano groups to the CoPc molecules (CoPc-CN). The resulting CoPc-CN/CNT hybrid material converts CO2to CO selectively with Faradaic efficiencies over 95% in a wide potential range and demonstrates extraordinary catalytic activity with a current density of 15.0 mAcm-2and a turnover frequency of 4.1 s-1at the overpotential of 0.52 V in 0.1 M KHCO3aqueous solution. The hybrid catalysts deliver high geometrical catalytic current densities comparable to the best heterogeneous catalysts while maintaining good per-site activity comparable to the best molecular systems for CO2electroreduction to CO. The study reveals that these molecule/CNT hybrid materials represent an attractive class of electrocatalysts for converting CO2emissions to sustainable fuels.
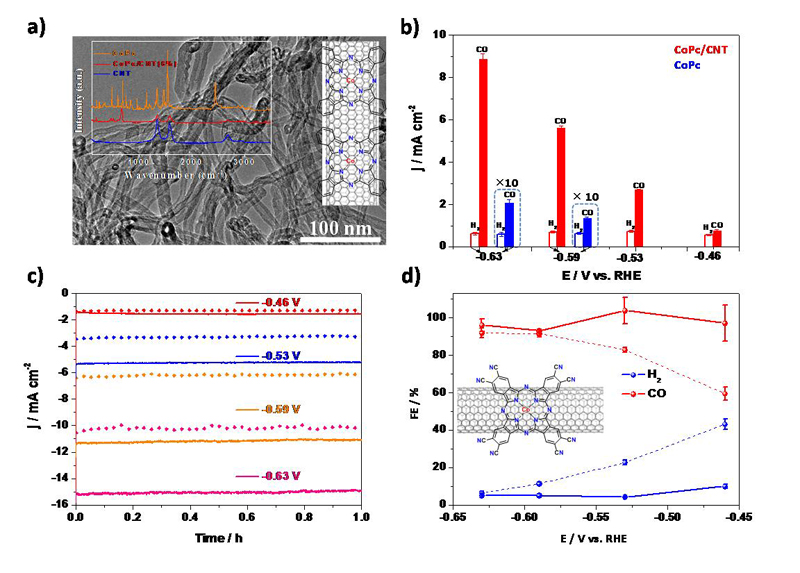
Fig 1. a) TEM image of the CoPc/CNT (6%) hybrid overlaid with schematic representation of the CoPc/CNT hybrid and Raman spectra of pure CoPc, CoPc/CNT (6%) hybrid and pure CNT;b) Faradaic efficiencies of CO2reduction gas products for CoPc/CNT (2.5%) (red) and CoPc (blue) at various potentials in 0.1M KHCO3aqueous solution;c) Chronoamperograms and d) Faradaic efficiencies of reduction products at various potentials for CoPc-CN/CNT(solid line)and CoPc-CNT(dotted line)in 0.1M KHCO3aqueous solution.
Xing Zhang, Zishan Wu (Yale University), Xiao Zhang and Liewu Li are the first authors with equal contributions. Other coworkers includeYanyan Li, Xiaoxiao Li, Haomin Xu, Xiaolu Yu,Zisheng Zhangand Prof. Hailiang Wang (Yale University). This work was supported by Shenzhen fundamental research funding (JCYJ20160608140827794), Shenzhen Key Lab funding (ZDSYS201505291525382) and Peacock Plan (KQTD20140630160825828).
Paper link:http://www.nature.com/articles/ncomms14675






