Recently, Prof. Yongye Liang’s research group has developeda multiscale structure-engineering approach to construct new hybrid electrocatalysts for water splitting with high performance. This work has been recently published in the journal“Advanced Functional Materials” entitled“Iron-Doped Cobalt Monophosphide Nanosheet/Carbon Nanotube Hybrids as Active and Stable Electrocatalysts for Water Splitting”.
Molecular hydrogen is regarded as an ideal energy vector. Electrochemical water splitting is apromising technique for centralized andcontrollable hydrogen production through the input of water and electric energy which could be converted from sustainable energy sources. In this regard, it is demanding to develop low-cost and high-performance electrocatalysts tofacilitate the cathodic hydrogenevolution reaction (HER) and anodic oxygen evolution reaction (OER) to improve the energy conversion efficiency.
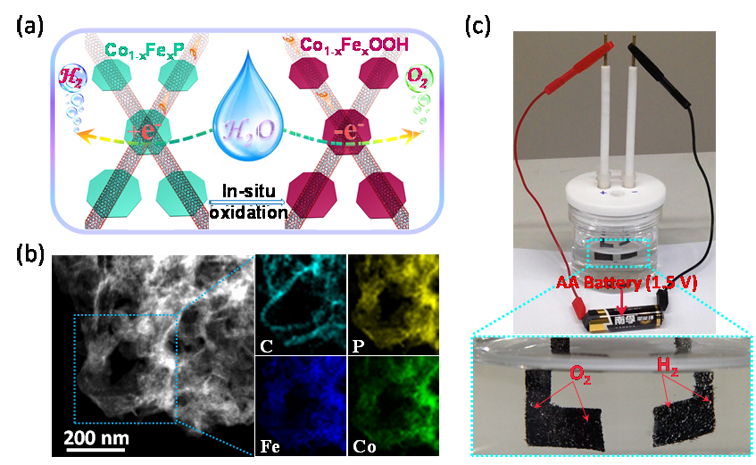
Fig. 1 (a) Schematic showing of the catalysts’ structure and the corresponding water splitting reactionsoccurred on these catalysts. (b) STEM image and EDX elemental mapping of the hybrids. (c) A constructed alkali-water splitting electrolyzer driven by a single-cell AA battery.
Prof. Liang and his co-workers developed a simpletwo-step method to synthesize CoP nanosheets with uniform Fe doping and hybridization with carbon nanotubes (Fig. 1a, b). The nanostructuring, uniform doping, and hybridization with CNT affordefficient electrocatalysts comparable to Pt/C for hydrogen evolution reactions in acidic, neutral, and alkaline electrolytes. It is found that the Fe doping level has different effects on catalytic activities in different electrolytes. Furthermore, after in situ oxidization/hydrolysis of the phosphides to corresponding oxyhydroxides, the hybrids exhibitexcellent catalytic performance for catalyzing the oxygen evolution reaction. A two-electrode alkaline water electrolyzer constructed with these hybrid electrocatalysts can afford a current density of 10 mA cm-2 at a voltage of 1.5 V, among the highest performance for water splitting electrocatalysts reported so far. This electrolyzercould also be driven by a single-cell AA battery (Fig. 1c).
Prof. Liang and Xing Zhang are the corresponding authors of this paper. Other co-authorsinclude Haomin Xu, Xiao Zhang, Zishan Wu (Yale University) and Hailiang Wang (Yale University).This work was supported byShenzhen fundamental research funding (JCYJ20160608140827794),Shenzhen Key Lab funding (ZDSYS201505291525382) and Peacock Plan (KQTD20140630160825828).
Paper link:http://onlinelibrary.wiley.com/doi/10.1002/adfm.201606635/full






