Radicals are inevitable intermediates during the charging and discharging of organic redox electrodes. The increase of the reactivity of the radical intermediates is desirable to maximize the capacity and enhance the rate capability but is detrimental to cycling stability. Therefore it is a great challenge to controllably balance the redox reactivity and stability of radical intermediates to optimize the electrochemical properties with a good combination of high specific capacity, excellent rate capability and long-term cycle life.
Recently, Prof. Zhouguang Lu from the Department of Materials Science and Engineering led his research team to make important progress in the electrochemical charge-discharge reaction mechanism of organic electrode materials. The results were published online in the Journal of the American Chemical Society (IF = 14.357). The paper was titled “Tunable Redox Chemistry and Stability of Radical Intermediates in 2D Covalent Organic Frameworks for High Performance Sodium Ion Batteries”.
The increasing demand of new energy storage systems has accelerated the development of organic compounds as potential alternative of current commercial metal oxides for battery electrode materials because of their intriguing merits of transparency, light weight, flexibility, abundant resource and sustainability. Up to now, organic compounds including organic carbonyls, organic radical compounds, porous polymers, polymeric Schiff bases, azo compounds, and graphdiyne have been explored for application in electrode materials. Generally organic compounds undergo radical intermediates formation and transformation upon charging and discharging. The radical intermediates usually exhibit high reactivity and fast kinetics, which is beneficial to improve the capacity and rate capability.
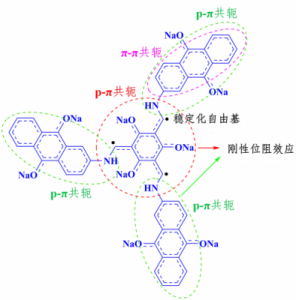
However, the highly reactive unpaired electrons of the organic radical intermediates are principally unstable and tend to combine with other active parts to form redox-limited dimer, resulting in the inactivation and the irreversible capacity loss of the electrode materials. The poor stability of the radical intermediates during the redox process is the central reason for the limited cycling life, largely restricting the development and application of organic electrode materials. Whereas, these radical intermediates function as active sites for charge storage. They should not be too stable to loss the electrochemical redox reactivities.
Controllably modulation of the stability of the radical intermediates is of vital importance to optimize the performance of the organic electrode materials. Previous reports of Professor Lu Zhouguang’s research group and other researchers have shown that conjugation interactions could contribute to delocalize the energy density of the unpaired electrons and the steric effect could restrict intermolecular electron self-exchange behavior. However, this simple introduction of the inactive aromatic units to provide conjugation interactions unavoidably results in reduction of specific capacities of the electrode materials. Hence, stabilizing the radical intermediates without the compromise of capacities decrease remains a great challenge.
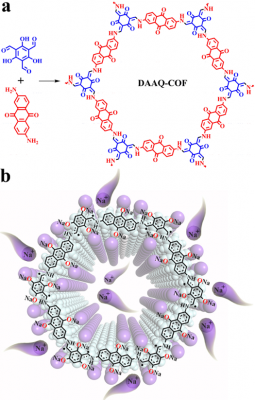
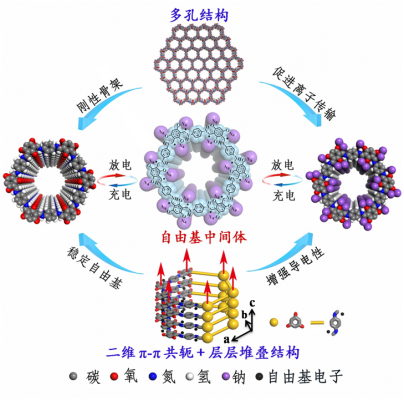
Two-dimensional covalent organic frameworks (2D COFs) have attracted increasing attention in energy storage due to their crystalline polymeric frameworks for restraining dissolution in electrolyte, π-conjugated skeletons for charge transportation, and abundant nanopores for ionic transportation. However, the state-of-art electrochemical performances of 2D-COFs are far from expectation. The research group of Professor Lu Zhouguang initially started working on COFs as a negative electrode material for sodium ion batteries. However, they discovered that they lacked the capacity and cycle stability they were looking for. The research group believed that when free radical intermediates were close to each other, it resulted in the inactivation of other free radical sites through an intermolecular reaction. Following an investigation by the Shanghai Synchrotron Radiation National Light Source, the team hypothesized that by controlling the stacking behavior of COFs 2D organic materials and reducing the stacking thickness, the intermolecular reactions between adjacent layers and free radical intermediates can be effectively inhibited. This would therefore regulate the reactivity and stability of free radical intermediates.
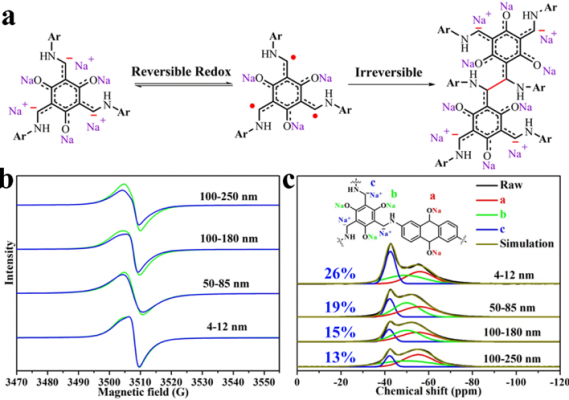
To test their hypothesis, the research group designed and synthesized 2D COFs of varying thicknesses (100-250 nm, 100-180 nm, 50-85 nm and 4-12 nm). By testing the electrochemical properties of each COF as a negative electrode for a sodium ion battery, the research group found an inverse relationship between the charge-discharge capacity and the thickness of the COF. The thicker materials had a lower energy density than the thinner materials. The group also found that there was a similar trend in stable capacity and number of cycles from thickest to thinnest materials. The results show that by reducing the thickness of the 2D electrode material, its capacity and cycle stability could be systematically and synchronously improved.
This paper provides new ideas for regulating the reactivity and stability of organic radical electrode materials, while providing an important reference for optimizing the performance of organic electrode materials.
This work was mainly completed by Mr. Shuai Gu and Mr. Shaofei Wu (co-first author), research assistants of Prof. Zhouguang Lu. Many members of the research team participated in the research work. The work of synchrotron radiation was completed in Shanghai, and was greatly assisted and supported by Dr. Zhenhua Chen, Dr. Lijuan Zhang, Dr. Wen Wen, Dr. Jingyuan Ma and Dr. Xiangzhi Zhang from Shanghai Light Source. The main characterizations in this work were conducted in Materials Characterization and Preparation Center (MCPC) of Southern University of Science and Technology (SUSTech) and Department of Materials Science & Engineering. The help on electron paramagnetic resonance (EPR) data collection and analysis from Dr. Yinhua Yang from MCPC is gratefully appreciated.
This work was financially supported by the Basic Research Project of the Science and Technology Innovation Commission of Shen-Zhen (No. JCYJ20170412153139454), the Guangdong Innovative and Entrepreneurial Research Team Program (No. 2016ZT06G587), and the National Natural Science Foundation of China (No. 21875097 and No. 21671096) and the special support from the President of Southern Science and Technology University.
Original paper: https://pubs.acs.org/doi/10.1021/jacs.9b03467






